Richard Labbé: Commitment to Excellence in Quality and Regulatory Compliance
In the dynamic landscape of medical device development, ensuring quality and regulatory compliance is paramount. At Technimount, Richard Labbé brings over 15 years of dedicated expertise in the field. As the Director of Quality and Regulatory Affairs, Richard’s role is pivotal in steering the company towards excellence in product quality and adherence to regulatory standards.
Leadership in Compliance and Excellence
 Richard’s journey into the realm of medical device development began with a strong academic foundation in engineering and nuclear research, which he skillfully translated into practical application within the medical device manufacturing industry. His specialization in medical device development, coupled with his proficiency in quality management and production transfer, positions him as a key figure in Technimount’s operations. His multifaceted expertise equips him with a comprehensive understanding of the intricacies involved in bringing innovative medical devices to market.
Richard’s journey into the realm of medical device development began with a strong academic foundation in engineering and nuclear research, which he skillfully translated into practical application within the medical device manufacturing industry. His specialization in medical device development, coupled with his proficiency in quality management and production transfer, positions him as a key figure in Technimount’s operations. His multifaceted expertise equips him with a comprehensive understanding of the intricacies involved in bringing innovative medical devices to market.
At Technimount, Richard spearheads the development and implementation of robust quality systems and processes, resulting in the achievement of ISO 13485:2016 certification for Technimount Medical. His meticulous approach ensures that all products meet stringent regulatory requirements while upholding the highest standards of quality and safety, spanning emergency medical services (EMS) and medicinal healthcare.
Upholding Standards for Safety in EMS and Medical Environments
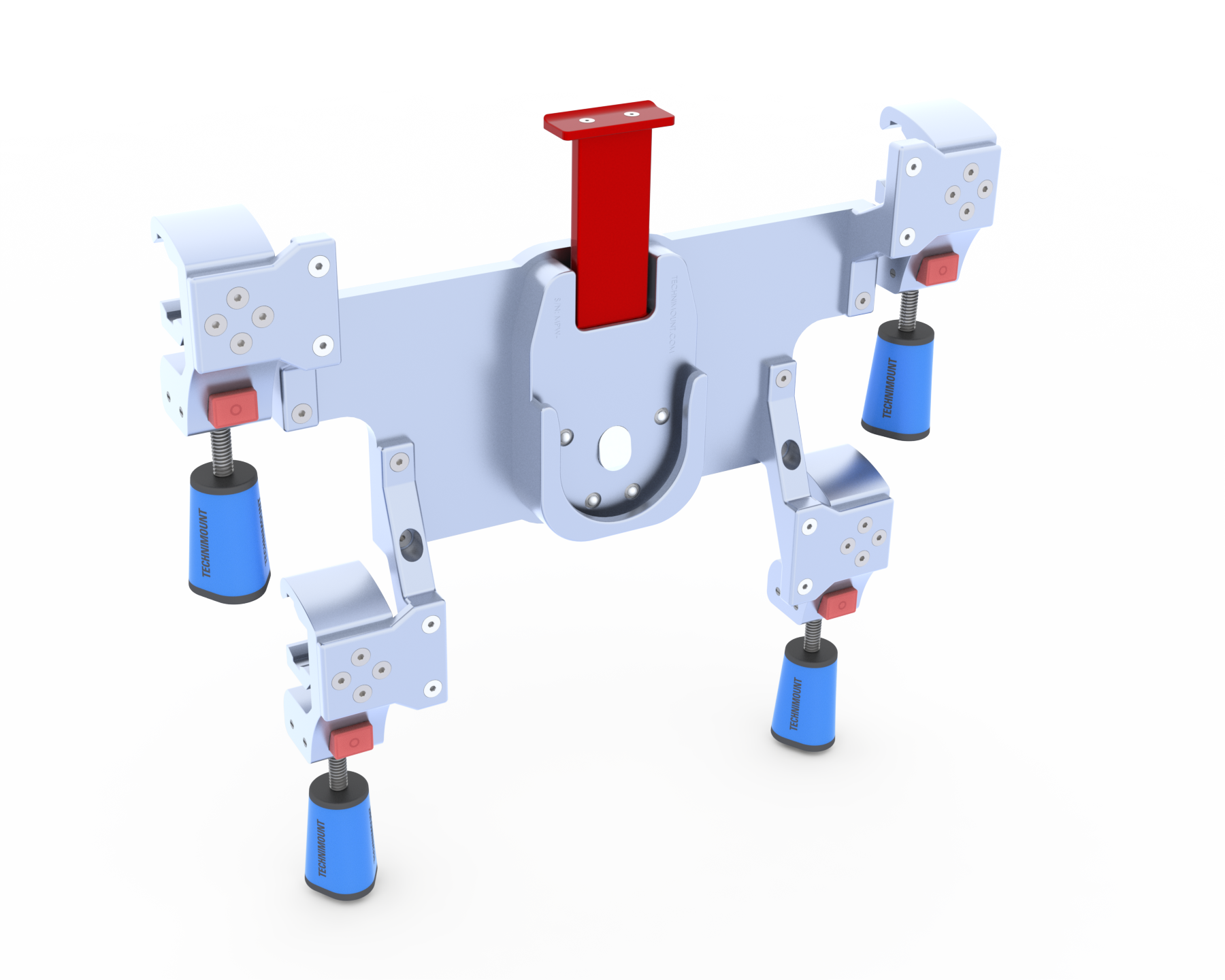
Richard’s role as Director of Quality and Regulatory Affairs is particularly crucial when it comes to testing Technimount’s solutions for adherence to SAE standards. Upholding these standards is paramount in the development of emergency vehicle mounts, as they serve as a benchmark for safety and performance. By meticulously overseeing the testing process in accordance with IEC60601-1 and SAE J3043 standards, he ensures that Technimount’s devices are rigorously evaluated to withstand challenging conditions and environments. This approach not only guarantees compliance with regulatory requirements but also underscores Technimount’s unwavering commitment to safety.
Richard’s commitment to regulatory compliance shines through in his efforts to fulfill the exacting standards of each country where Technimount distributes its products. This encompasses adherence to a comprehensive array of regulations, including but not limited to the US-FDA Establishment license requirements, FAA air transport certification, Health-Canada Medical Device Regulation, European CE mark, and United-Kingdom UKCA mark.
Beyond his responsibilities in quality and regulatory affairs, Richard plays a role in the product development process, providing guidance and support to cross-functional teams. His keen eye for detail and strategic foresight contribute to the seamless execution of projects. Furthermore, he also manages the company’s patent and trademark portfolio, safeguarding Technimount’s innovations and intellectual property. Richard Labbé’s leadership is marked by integrity, innovation, and a relentless pursuit of excellence, fostering a culture of quality and compliance. His expertise guides the company through the complexities of the medical device industry, driving Technimount towards sustained growth and success.



 < ZOLL X Series
< ZOLL X Series