Safety Standard for Ambulance Equipment Mount Devices
Patient transportation is a critical aspect of the healthcare industry which requires the use of portable medical devices to maintain efficient patient care. A reliable mounting system is responsible for securing equipment whose users require access to its readings and controls in chaotic, emergency situations. In the event of a collision, weak and poorly designed mounting systems represent significant risk factors, potentially becoming sources of serious injuries, lawsuits, and damaged or broken equipment. Thus, to help guide manufacturers, OEM’s and EMS operators, the Society of Automotive Engineers (SAE) developed a rigorous industry standard to test systems that secure medical devices during emergency transport: SAE-J3043.
Understanding SAE J3043
SAE J3043 provides guidelines for testing the performance of mounting systems under various crash scenarios involving emergency vehicles. This recommended standard describes the dynamic and static testing procedures required to evaluate the integrity of device mounting systems when exposed to frontal or side impacts (i.e., crash impacts.) Compliance with SAE J3043 ensures that vehicles have sturdy and reliable mounting systems that can resist the extreme forces generated during crash events, dramatically reducing the risk of injuries to anyone onboard the ambulance and the risk of damaged equipment.
Importance of SAE J3043
SAE J3043 is critical for emergency vehicle safety as it outlines a mount’s performance requirements through standardized testing methods that evaluate its effectiveness in a crash event; SAE-compliant systems have become crucial factors in modern ambulance safety. Numerous Federal and state regulators have implemented vehicle specifications, which themselves refer to SAE’s verification methods.
Testing Process
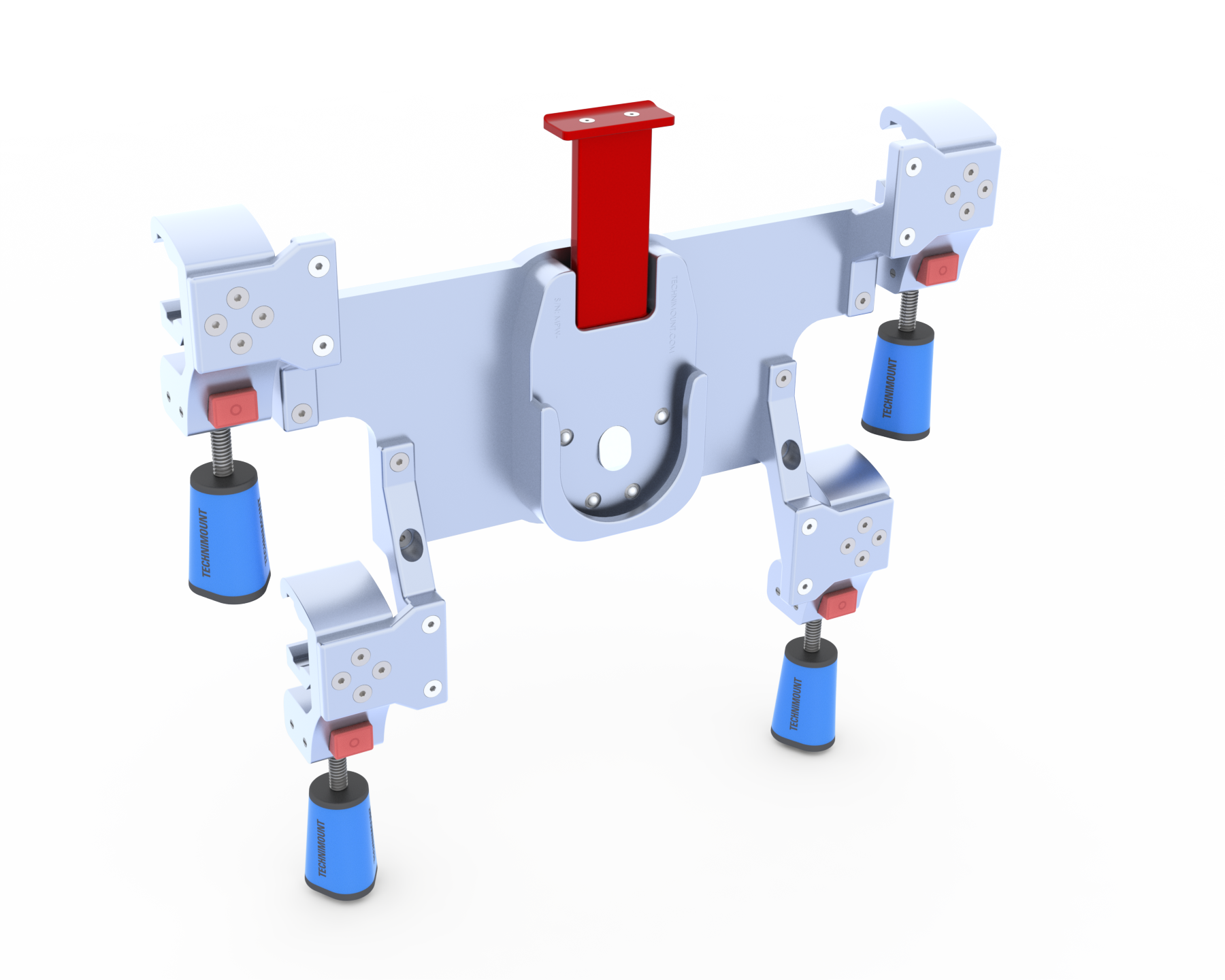
At Technimount EMS, we take pride in creating innovative and high-quality products that exceed industry standards. Our design process involves brainstorming multiple concepts to meet customer requirements, conducting computer simulations, and creating prototypes for in-house testing, all in compliance with SAE J3043’s recommended protocols.
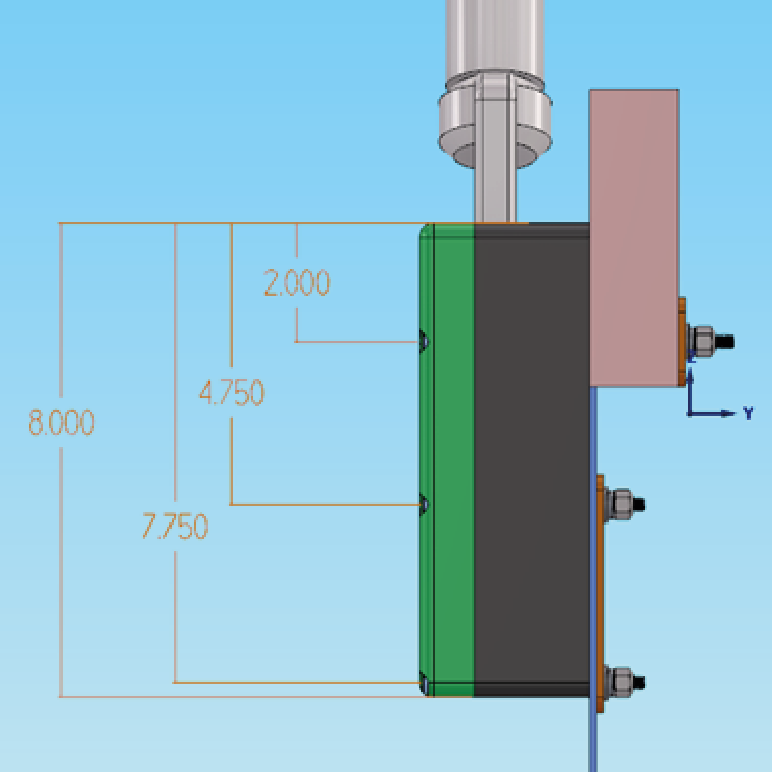
Step 1: Conceptualization
Our R&D team brainstorm multiple design concepts to meet specific requirements for different brands and types of medical devices.
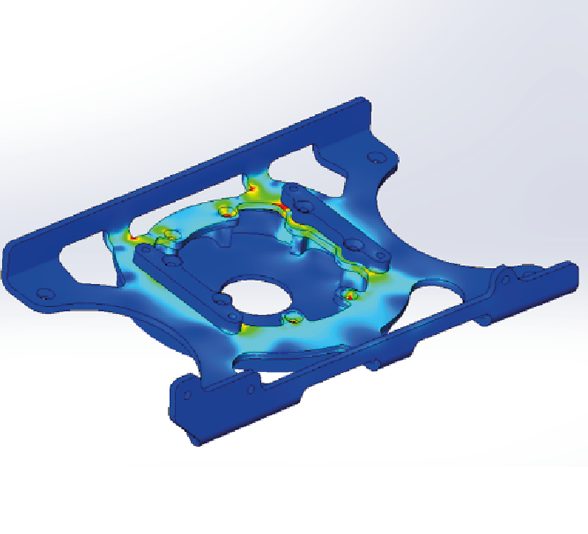
Step 2: Computer Finite Element Analysis (FEA) Simulations
The team uses advanced computer simulations to test the material, density, hardware, size, and other critical variables. These simulations also help evaluate mechanical strength in different stress environments to ensure the design can withstand real-world conditions.

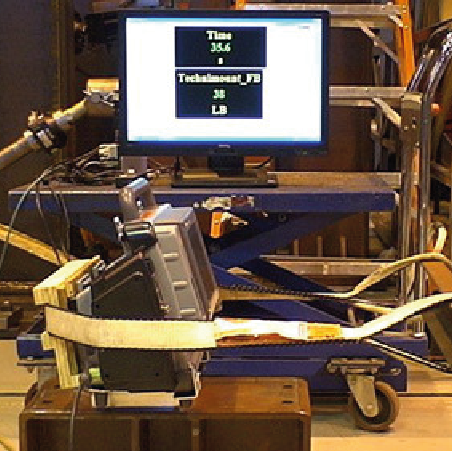
Step 3: In-House Prototype Testing
We manufacture and test prototypes to validate our data using a variety of in-house testing methods.
Step 4: Final Certification
Our products are certified according to the SAE J3043 recommended practice by Technimount Engineering using production equivalent products. This final certification provides external validation and confirms that our products meet or exceed these standards.
Compliance with SAE J3043 guarantees that medical devices will safely accompany patients and medical personnel in emergency interventions. Technimount EMS is committed to a thorough product development process whose standards prioritize innovation and reliability; we base our approach on the belief that there are no shortcuts to safety.